
The 21st century is going to bring massive changes to the medical device development sector and the role of computational modeling and simulation-based medical device development is expected to increase further.
In silico studies (physics based computational modeling and simulation) are already being used to virtually test medical devices.
This blog will address how the medical device industry can ride the wave of technological progress and what the future holds for medtech innovation.
How can technologies be utilized in design and development of medical devices?
Computational Modeling and Simulation for Medical Device Development
Traditionally, medical device development has been a long and painstaking process. Building and testing prototypes takes a lot of design effort and resources. Medical device development relies heavily on evidence gained from bench experiments, animal testing and clinical trials.
Hence, the time to market has been very long for medical devices. The current application of CMS in gathering evidence for medical device development is less than 15 percent [1]. It can be envisaged that this will increase in the future to more than 50 percent of the development process [1].
CMS can provide an alternative to traditional methods of testing by providing alternative testing capabilities and reducing reliance on bench testing, animal trials and clinical trials. It can greatly expedite product development as most designs can be filtered out at the design stage while leading designs can be tested virtually prior to prototyping.
CMS has the capability to create thousands of virtual designs and situations before building the first physical prototype. It can support “fail fast” device development, reduce time to market and provide an impetus for development of disruptive and innovative technologies.


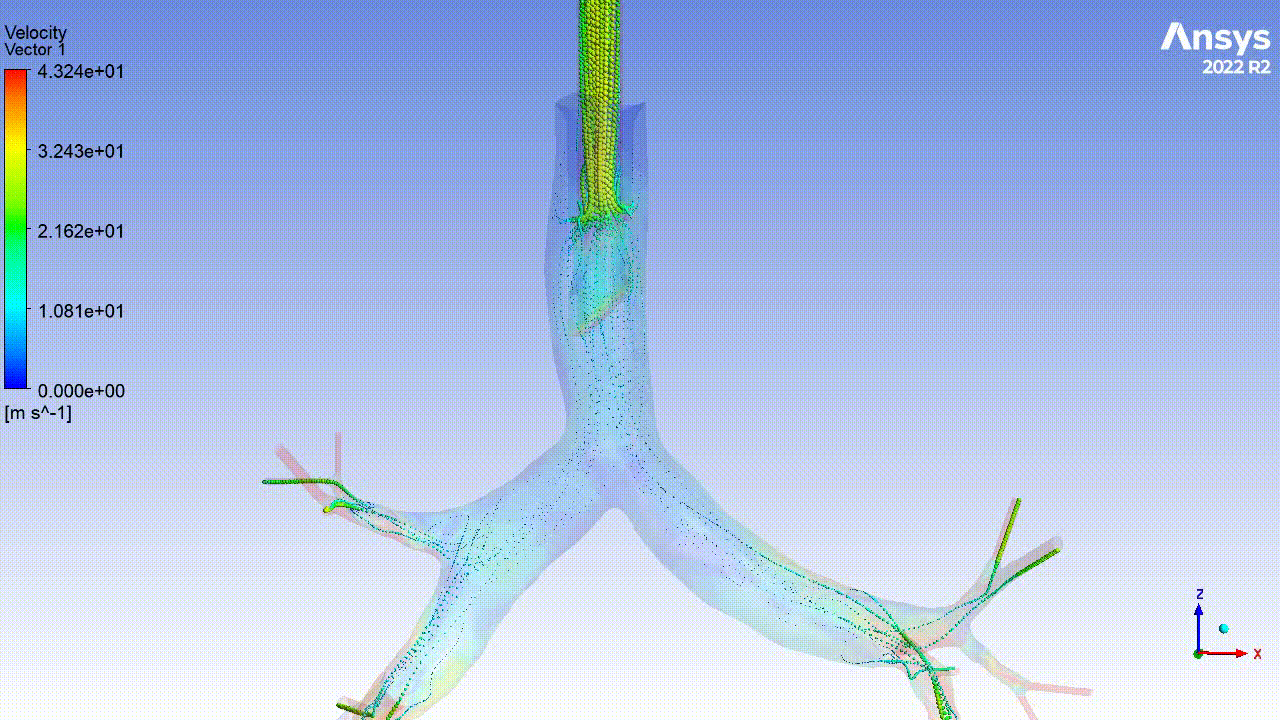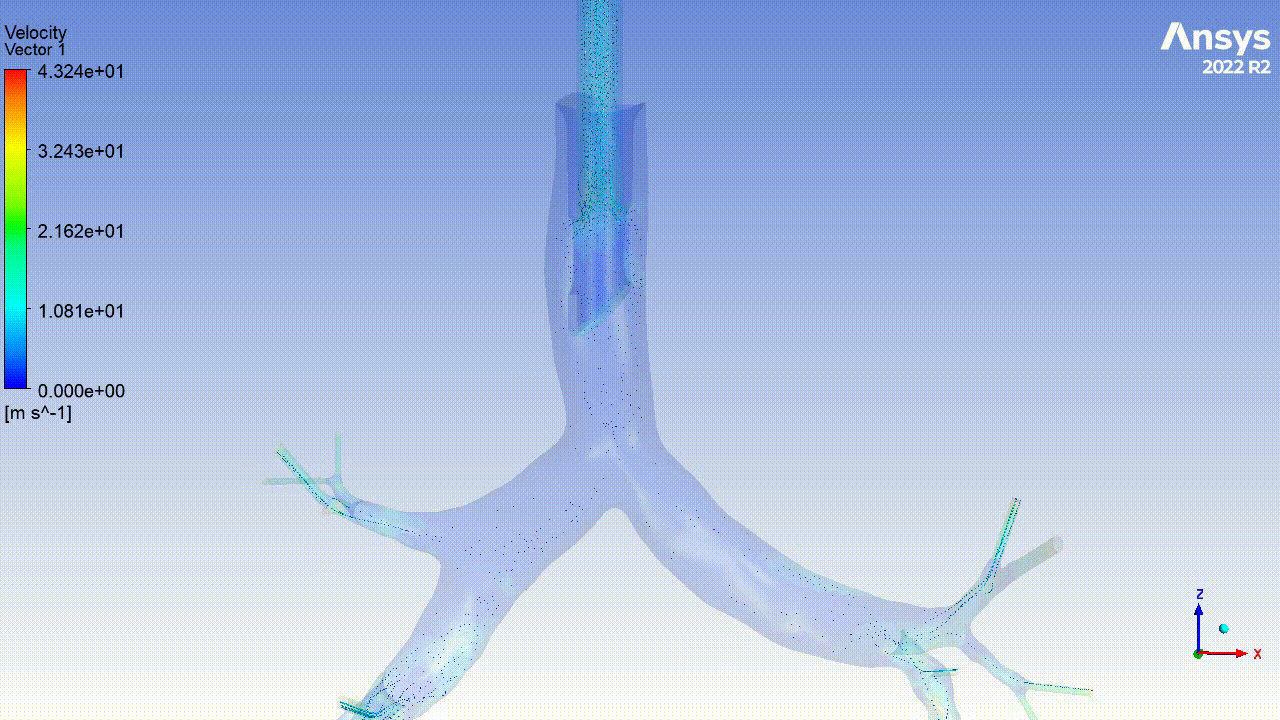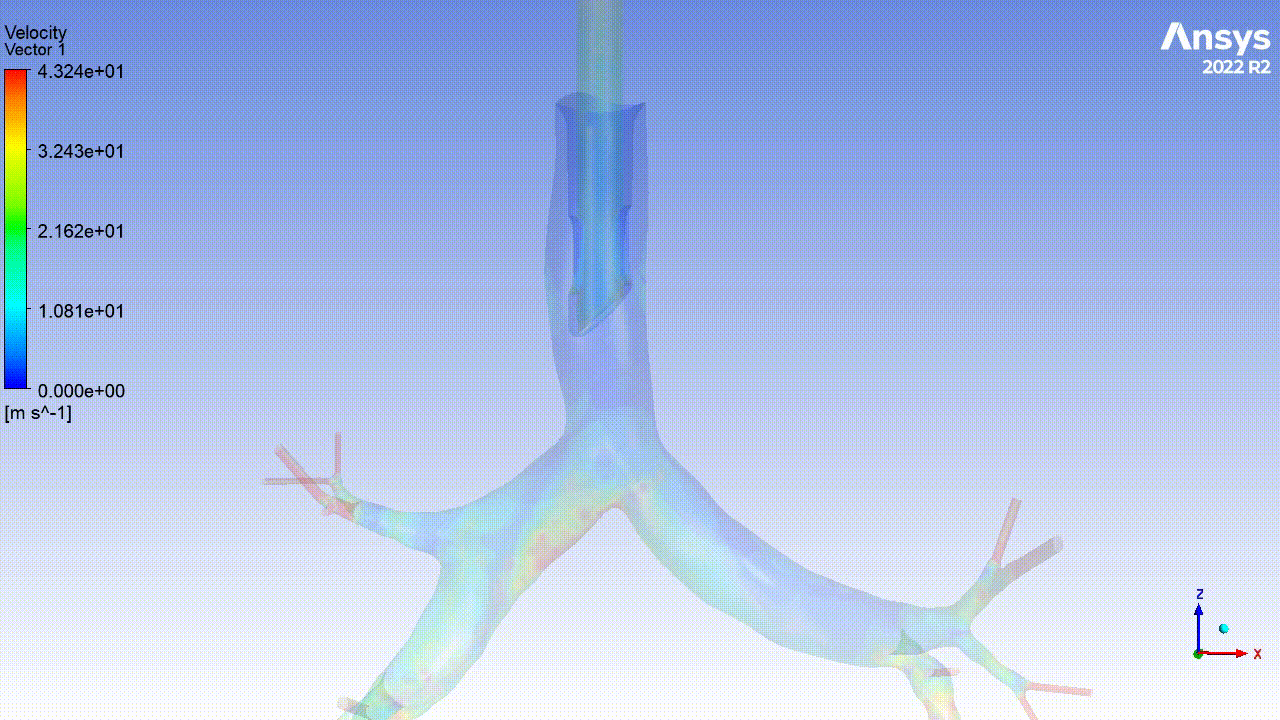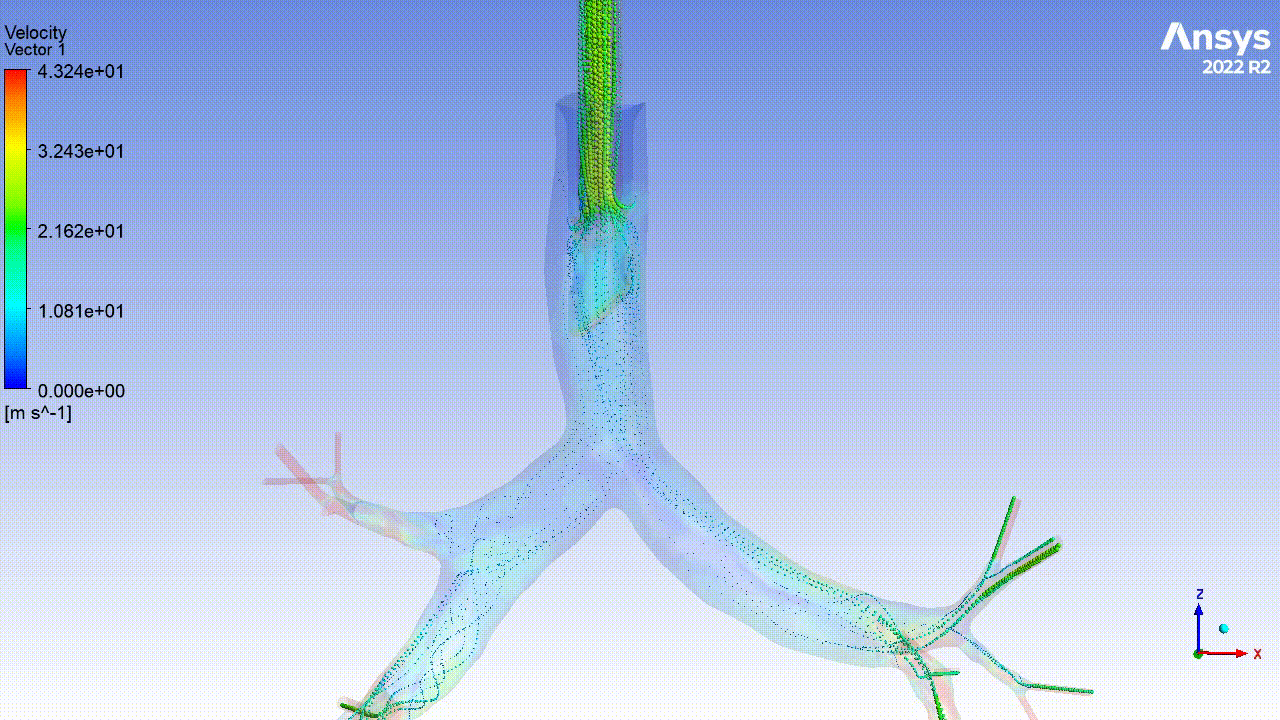
Currently, CMS is being used to model device, anatomy, physiology, toxicology, and treatment outcomes for clinical trials. For example, coronary artery bypass can be simulated, and its efficacy can be readily verified.
The fluid flow patterns generated by a catheter used in mechanical ventilation can provide information about the performance of the catheter mechanical design. A heart valve can be implanted in a virtual patient model, and its performance can be evaluated to pre-emptively assess any risks associated with the implantation. A coronary stent can be deployed, and fatigue assessment can inform whether the stent will fail under physiological conditions over its expected lifetime.
The role of CMS in total product life cycle for medical devices is ever increasing in aspects such as design initiation, prototyping, redesign, optimization, risk and failure analysis and it is going to increase further in future [2]. Regulatory bodies are already recognising this huge potential and shift in the market. FDA has identified the huge potential of in silico studies and made it one of its priority areas in their 2018 strategic policy roadmap.
The vision of the regulatory bodies is to encourage and normalize the use of simulations in medical device development. In pursuit of that, FDA also included simulation in its strategic policy roadmap, Medical Innovation Access Plan, which includes “Advance the use of in silico techniques to develop novel methods for creating models of virtual patient outcomes and modernizing FDA’s evaluation of patient benefit and risk”.
Outlook of the Future Virtual Medical Device Development Ecosystem
Physics based simulation is a very powerful tool that provides insight into the physical phenomena where risky interventions can be simulated in advance to mitigate the risks involved. However, its use has been limited to subsystem or organ level.
Virtual models of body organs have been developed and digital evidence of medical device efficacy has been obtained by solving the mathematical equations that govern the physical processes.
Rapid advancement in technology has provided us the capability to process huge amounts of data and, with the advent of disruptive technologies such as quantum computing, this capability is going to improve further. Physics-based simulations provide digital evidence by solving increasingly complex biological problems that were not possible previously.

The question we need to ask ourselves is: In the wake of all these developments, what does the future hold for computational modeling and simulation application within the medical device development and healthcare?
This question is not easy to answer but we can think on the following lines that paint a broader picture of what to expect in future.
In terms of simulation-based medical device development, thousands of designs can already be generated in short periods of time and potential designs can be refined and perfected. The effect of materials, geometric parameters, chemical composition, concentrations can be assessed at early stages of the design.
The next stage will be to test these novel designs on VIRTUAL patients. A virtual patient will be a digital avatar that is representative of a typical patient based on the data obtained from thousands or millions of real patients. This will enable researchers to generate patient models based on race, size, gender and age of interest for a particular study and test the efficacy of the device for the target population.
This will completely disrupt the way medical device companies perceive clinical trials.
Imagine the impact if we had the ability to test different ventilator designs on virtual Covid-19 patients. Companies would not have had to go through lengthy and clinical trials on patients incorporating biological variability to prove the efficacy of their devices.
In a nutshell, a medical device development ecosystem comprised of virtual patients, medical device development and diagnostics/treatments can become a reality in the near future.
This will speed up medical device development in a more comprehensive way by shortening the time to market, lowering the cost and providing an impetus for development of innovative and disruptive technologies.
In the distant future, the virtual patient concept will be extended further to “Digital Human”. While Virtual Patients allow testing of medical devices in typical models, a “Digital Human” twin will tackle the concept of individual medical device development head on for personalized medicine.
A Digital Human is a living replica of a real human with the ability to “learn, adapt and improvise” based on the data received from the subject. In order to develop a reliable digital human, quality data with resolution varying from cell level to human body level, needs to be collected and Big Data technologies need to process large amounts of data.
Physics based models will provide the basic framework for digital human. Reduced models can be generated using AI or VR that provide much faster results.
In terms of medical device development, an ultimate digital development platform would incorporate personalized medical needs. It will be able to provide much comprehensive information that is not yet possible. For instance, it will be able to predict the long-term effects of the device, how it responds to changing environments, and predict its failure.
To summarize, in the wake of all the technological advancements, we need to be aware of these new challenges and brace ourselves for the bright future ahead.
[1] https://www.fda.gov/science-research/about-science-research-fda/how-simulation-can-transform-regulatory-pathways [2] https://www.fda.gov/media/163156/download
Title Image: Photo 80865858 © Agsandrew | Dreamstime.com
Other Images: StarFish Medical
Muhammad Jamil, PhD, is a Computational Fluid Dynamics (CFD) Engineer in the Design and Analysis group at Starfish Medical. As part of the design and development team, he specializes in using Computational Modeling and Simulation in making design decisions and to accelerate the medical device development.






