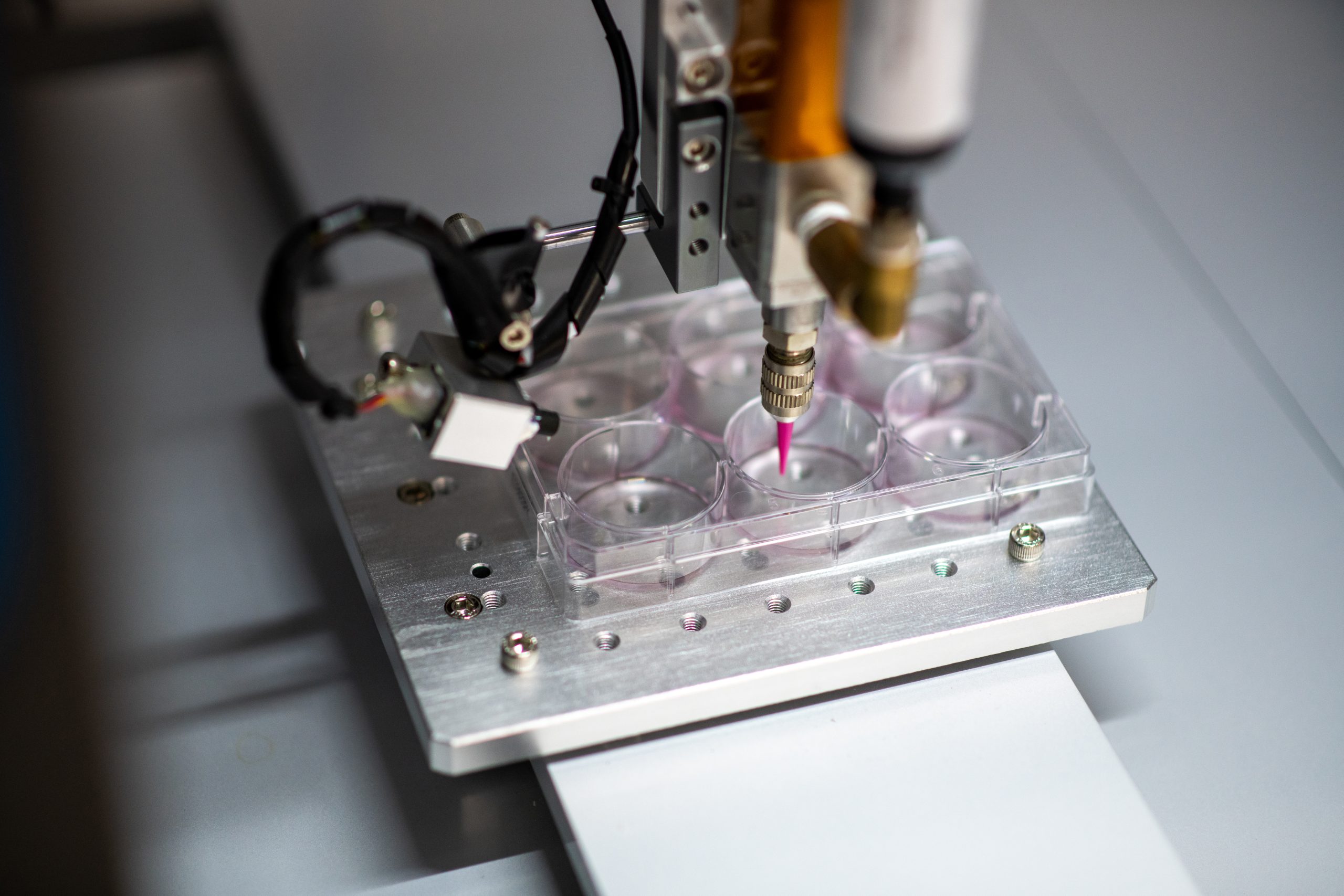
Bioprocessing (also known as biologics manufacturing) is a branch of the medical industry where bacteria, cells or enzymes are used to create and isolate biopharmaceutical products.
This is broadly accomplished in multiple upstream (e.g., cell culture, cultivation and harvesting) and downstream (e.g., filtration, centrifugation and chromatography) unit operations in concert with process analytical technology.
These operations are performed with specialized equipment that has been designed to satisfy the unique requirements of biologics manufacturing.

Simple bioprocess flow diagram with major unit operations for therapeutic protein production. (StarFish Medical)
Biopharmaceuticals, also known as biologics, are drugs made by or extracted from living organisms. These products encompass an ever-growing list of compounds, including monoclonal antibodies, enzymes, nucleic acids, vaccines, cell therapies and gene therapies.
Since the introduction of the first product approximately 40 years ago, biologics sales have grown to dominate the pharmaceutical sector with biologic products making up the lion’s share of every top selling drug list over the last decade.
Growth in the biologics market has largely been driven by the development and improvement in manufacturing processes and technologies, collectively referred to as bioprocessing. In particular, the introduction of single-use technologies, continuous processing, as well as better analytical tools and sensors are some of the technologies that have changed bioprocessing over the past decade.
Drivers for Innovation
While platform processes and equipment have been developed over the last 40 years to produce relatively mature products like monoclonal antibodies, the need for new technology development has and continues to be driven by changes in the market landscape. These drivers include: the introduction of new therapeutic classes, increasing manufacturing productivity/reducing cost, reduced batch size requirements, and increased digitalization and automation.
New Therapeutics
Perhaps the greatest current driver for bioprocess equipment innovation is the development of cutting-edge cell and gene therapies for the treatment or cure of a range of debilitating conditions including cancer, neurodegenerative diseases, and bleeding disorders. The current manufacturing processes for these products are generally scaled-up versions of small-scale, complex and highly manual laboratory processes.
These methods may be suitable for the limited production scales needed to support clinical testing or market entry for small patient populations (i.e. rare and orphan diseases), but are unlikely to suffice for products intended for larger patient populations.
In other cases, such as autologous (Chimeric Antigen Receptor) CAR T-cell therapies or personalized medicine, improvements in manufacturing equipment that reduces or eliminates manual intervention are essential to improve product reliability and drive down cost. Accordingly, there is significant activity in this sector around the development of new bioprocess equipment suitable for cell and gene therapies.
Advances in bioreactor design, cell culture process analytics and control, development of closed, single-use equipment and novel purification tools are all areas of ground-breaking and exciting advances showing significant market growth.
Manufacturing Productivity
Since the advent of biopharmaceuticals, there has been an on-going effort to increase productivity in biomanufacturing. This trend is broadly driven by the continuous need to drive down production costs and reduce production facility footprints (with associated capital costs).
Bioprocess intensification, in which equipment & processes are made more efficient and compact, is how increased manufacturing productivity is realized. Examples include smaller bioreactors using more productive cell cultures, and high productivity membrane chromatography devices that enable significant equipment downsizing.
In addition, the explosion in the use of single-use bioprocess technologies has improved productivity by eliminating non-productive activities like equipment cleaning & validation, reduced personnel requirements and shortened change-over times between batches.
For these reasons, innovation in bioprocess equipment design centered on downsizing and new and improved single-use components is expected to continue.
Small Batch Manufacturing
Experts predict an increase in the number of biopharmaceutical products marketed, with a focus on developing smaller markets, personalized products, and biosimilars. Accordingly, an industry trend of designing and building smaller, more flexible manufacturing facilities is currently underway and the growth of gene and cell therapies has reinforced this broader trend.

Aspen Surveys, Aspen Alert Jan. 27, 2023, Aspen Media Inc.
Novel biotherapeutic manufacturing platforms utilizing innovative technologies like microfluidics are being developed for the production of gene therapies and vaccines in compact and mobile “factories” that allow distributed manufacturing networks to enable products to reach patients more quickly.
Proponents of personalized medicine are working towards a future where therapeutic manufacturing can be automated and scaled down sufficiently to enable the placement of biomanufacturing “vending machines” in hospitals and clinics.
Biopharma 4.0
The Biopharma 4.0 concept has been steadily gaining momentum over the past several years as a vision for the factory of the future. The factory of the future is one where “smart” processes and equipment continuously collect and learn from data to predict & and maintain optimal operating states.
The high degree of automation should reduce the number of skilled operators needed while making manual tasks less error-prone and more efficient, freeing scientists to work on multiple processes simultaneously and respond rapidly to process deviations, even from remote locations. Innovation in process analytical technologies, more sophisticated digital control systems (such as artificial intelligence and machine learning) and standards for equipment integration are all areas of intense development to help realize Biopharma 4.0.
At StarFish, we understand the evolving needs of the biomanufacturing industry. Accordingly, we’ve assembled the resources and knowledge needed to help our clients develop the next wave of bioprocess equipment and tools needed to enable this exciting vision for the biofactory of the future.
Summary
Bioprocessing needs to become faster, more portable, more affordable, and more accessible to deliver on the full promise of current and future biopharmaceuticals for as many patients as possible. These necessary improvements can only be realized by accelerating innovation in bioprocess equipment design, control and integration.
Gary Skarja is a Bio Services Program Manager at StarFish Medical. Over his 23-year career in the commercial life sciences sector, he has led the development of new medical materials & devices, regenerative medicine technologies and single-use biomanufacturing tools at companies ranging from pre-revenue start-ups to a multibillion dollar global organization. Gary holds a B.Eng. and M.Eng. in Chemical Engineering from McMaster University and a Ph.D. in Chemical Engineering from the University of Toronto.






